Get Free Sample
1. Morphological Structures:
SAP can be categorized as powders, particles, fibers, spheres, emulsions, and membranes. These structural variations are designed to meet diverse application requirements. For example, powders can be used in the production of sanitary napkins and diapers by incorporating them into multi-layered sheets. Spherical products and particles are suitable for deodorant applications. Fiber-based SAP are used to create antistatic electric fibers, while membranes find use in anti-frost sheets. Emulsion forms are preferred for soaking and painting applications.
2. Sources:
SAP can be classified into three main categories based on their sources:
a. Natural macromolecules, including polysaccharide-based and polypeptide-based materials. Polysaccharide-Based SAP, such as cellulose, starch, chitin, chitosan, and natural gums (e.g., xanthan, guar, and alginates), can be prepared through direct cross-linking of polysaccharides or by graft copolymerization of vinyl monomers onto polysaccharides in the presence of a crosslinking agent [1].
b. Semi-synthetic polymers.
c. Synthetic polymers, such as petrochemical-based.
Compared to synthetic counterparts, natural-based SAP offer several advantages, including degradability (reducing environmental pollution), compatibility, non-toxicity, and cost-effectiveness [2]. However, petrochemical-based SAP have encountered challenges such as rising global oil prices and environmental pollution concerns. One drawback of natural polymers is their low solubility in water, necessitating chemical modifications to create soluble derivatives like carboxymethyl starch (CMS) and carboxymethyl cellulose (CMC) [3].
3. Preparation Methods:
SAP can be categorized based on preparation methods into crosslinking polymerization, radiation cross-linking, graft polymerization, and the formation of water-soluble polymer networks [4].
4. Electrical Charges on Cross-linked Chains:
A. Non-ionic polymers: They absorb water and aqueous fluids through a mixing effect due to hydrophilic groups, resulting in a swollen, soft gel due to hydrogen bond solvation [5].
B. Ionic polymers: These include anionic and cationic polymers. The presence of charges along the backbone significantly increases swelling due to strong ion-dipole interactions and osmotic pressure enhancement from solvated counter-ions.
C. Ampholytic (amphoteric) polymers: These contain both basic and acidic groups.
D. Polybetaines (zwitterionic): They contain both cationic and anionic groups in each repeating unit [4]. Recently, photochromic hydrogels were developed with a water absorption capacity of 2800 g/g.
SAP incorporated into the soil can absorb water from rainfall or excessive irrigation. They then release this water slowly based on root demand through osmotic pressure differences, leading to improved growth rates. Additionally, SAP can be utilized for the controlled release of pesticides and agrochemicals. SAP have a significant impact on various soil properties, including permeability, aeration, density, structure, erosion resistance, evaporation, microbial activity, and infiltration rates. They are also used to enhance nutrient retention and water-holding capacity in sandy soils (macro-porous medium), particularly in arid regions. Furthermore, SAP can reduce water losses, irrigation frequency, and soil compaction tendencies.
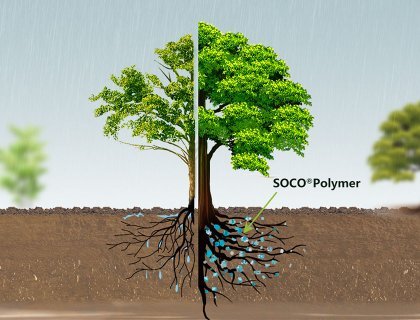
In terms of plant health, SAP can enhance color intensity, coverage percentage, sport turf density, and reduce wilting levels. In agriculture, SAP are mixed with soil, seeds, agricultural chemicals, and fertilizers, creating water-absorbent slow-release fertilizers. In forestry, SAP are applied over roots to protect trees from drying out during transportation.
Strengthening of Concrete Application:
Despite the popularity of mortar and concrete as construction materials, they suffer from certain weaknesses such as delayed hardening, significant drying shrinkage, low chemical resistance, and low tensile strength.
SAP can play a crucial role in addressing these challenges by acting as an internal water source during the hydration process, following this mechanism:

1. SAP absorb water molecules from the fresh concrete mixture and release them later when the relative humidity decreases due to the hydration process.
2. As SAP reach their final sizes, they form stable, water-filled inclusions. Water is then drawn from these inclusions into smaller capillary holes and utilized by the cement hydration process.
SAP are used to facilitate internal curing of concrete, thereby mitigating autogenous shrinkage. Internal curing allows for curing "from inside to outside" by creating internal reservoirs that provide water throughout the mix, unlike external curing that only penetrates a few millimeters. Additionally, the gel created by SAP can improve workability and stability by cushioning and lubricating the concrete mass.
Key properties of effective mortar include consistency and setting times:
Consistency refers to the water content of the paste needed to achieve the desired consistency.
Setting refers to the transition from a fluid to a rigid state, mainly caused by the hydration of tricalcium silicate (C3S) and tricalcium aluminate (C3A), occurring in initial and final stages.
Setting is crucial in concrete work to maintain fresh concrete plastic long enough for transportation, casting, and compaction [6]. The initial set time is defined as the time from mixing water with cement until penetration no deeper than 4-6 mm from the bottom, with a minimum of 45 minutes according to ASTM C 150-09. Final setting time is typically measured from the moment water is mixed with cement, with no specific limits in European or ASTM standards [7].
Other Applications:
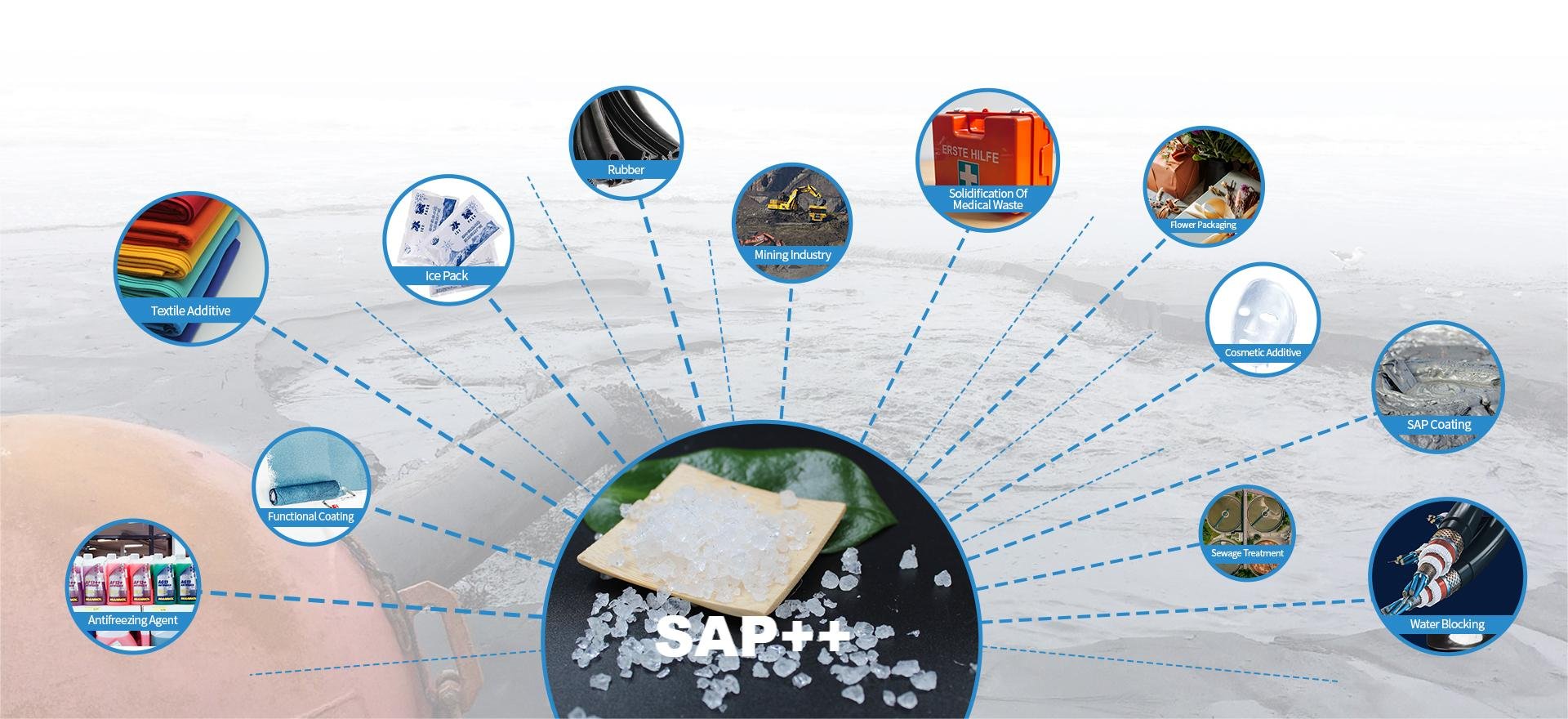
SAP can also be used in many applications such as flame retardants, dehydration of adulterated fuels, artificial snow, ice packs, decorative (colored) products, sanitary napkins, building interior decoration, waste solidifiers in sludge treatment, drilling fluids, medical waste solidifiers, nursing pads, etc.
[1] A. Mathur, S. Moorjani, and A. Scranton, "Methods for synthesis of hydrogel networks: A review", J Macromol Sci-Rev Macromol Chem Phys, Vol. 36, pp: 405-430, 1996.
[2] W. Wang, J. Zhang, and A. Wang, "Preparation and swelling properties of superabsorbent nano composites based on natural guar gum and organovermiculite", Applied Clay Science, Vol. 46, pp: 21–26, 2009.
[3] A. Li, J. Zhang, and A. Wang, "Utilization of starch and clay for the preparation of superabsorbent composite", Bioresource Technology, 98, 2007.
[4] D. Gao, "Superabsorbent Polymer Composite (SAPC) Materials and their Industrial and High-Tech Applications", 2003.
[5] F. Jaber, "New Routes for Synthesis of Environmentally Friendly Superabsorbent Polymers", M.Sc. Thesis, An-Najah National University, 2012.
[6] M. Daoud and M. Al-Nasra, "The Use of Super Absorbent Polymer as a Sealing Agent in Plain Concrete", American Journal of Engineering Research, Volume 03, Issue 03, pp 132-137, 2014.
[7] M. Neville and J. J. Brooks, "Concrete Technology", Second edition, 2010. 31-L. Davies, J. Novais, and S. Martins-Dias, "Detoxification of olive mill wastewater using superabsorbent polymers", Centre of Biological and Chemical Engineering, Instituto Superior Técnico, Portugal, 2009.
We have “Ask The Expert” online service 24/7. If you have any questions please contact us.